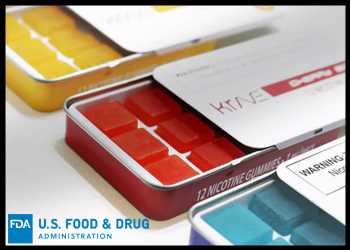
The U.S. Food and Drug Administration has warned against marketing illegal flavored nicotine gummies, manufactured by VPR Brands LP, doing business as, “Krave Nic”.
The warning letter was issued as the agency considers these gummies as public concern due to their resemblance to kid-friendly food or candy products and the potential to cause severe nicotine toxicity or even death among young children.
It was the first warning letter for this type of product, FDA noted.
The company has not submitted a premarket tobacco product application or PMTA, and does not have a marketing authorization order to manufacture, sell or distribute these products in the U.S.
In its letter, the agency has asked that the firm must not sell or distribute violative products. The company website now shows the product has been discontinued.
Krave Nic marketed gummies that have 1 milligram of nicotine each with 12 gummies per tin, and were available in three flavors – Blueraz, Cherry Bomb and Pineapple. The packaging claims that the products contain tobacco-free nicotine.
As per research, ingesting 1 to 4 milligrams of nicotine could be severely toxic to a child under 6 years of age depending on the child’s body weight. For youth of any age, nicotine toxicity may lead to nausea, vomiting, abdominal pain, increased blood pressure and heart rate, seizures, respiratory failure, coma and even death. Nicotine is also highly addictive and exposure during adolescence can harm the developing brain.
A recent study published in the journal Pediatrics showed that flavored non-tobacco oral nicotine products, including gummies and lozenges, were among the most commonly used tobacco product among youth in southern California – second only to e-cigarettes.
FDA Commissioner Robert Califf said, “Nicotine gummies are a public health crisis just waiting to happen among our nation’s youth, particularly as we head into a new school year. We want parents to be aware of these products and the potential for health consequences for children of all ages – including toxicity to young children and appeal of these addictive products to our youth. The FDA will not stand by as illegal products infiltrate the marketplace.”
The warning letter requests a written response from the manufacturer about its plans to address any violations and bring their products into compliance with the Federal Food, Drug, and Cosmetic Act or FD&C Act.
For More Such Health News, visit rttnews.com
Source: Read Full Article