Shares of Talaris Therapeutics Inc. (TALS) have lost 64% from their 52-week high of $19.82 last June, and trade around $7.
Talaris is a late-clinical stage cell therapy company developing a novel, single-dose, investigational cell therapy with potential to transform standard of care in solid organ transplantation and multiple severe immune and non-malignant blood disorders.
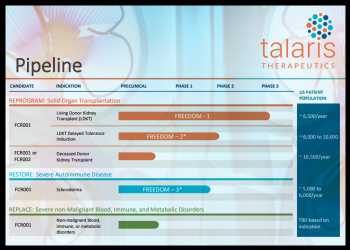
The lead product candidate is FCR001, under a phase III trial to induce durable immune tolerance in living donor kidney transplant (LDKT) recipients, dubbed FREEDOM-1.
This study is designed to enroll 120 first time, adult living donor kidney transplant (LDKT) recipients, randomized 2:1 between FCR001 and standard of care (SoC). The primary endpoint is the proportion of FCR001 recipients who are free from chronic immunosuppression, without biopsy proven acute rejection (BPAR), at month 24 post-transplant.
As of October 2021, five patients were dosed with FCR001 in the FREEDOM-1 trial.
Initial data reported from this study, last November, were promising. According to the company, all three evaluable patients treated with FCR001 following kidney transplant demonstrated more than 50% chimerism at 3-, 6- and 12-month timepoints post-transplant, and two patients who were transplanted and dosed more than 12 months prior had successfully discontinued the use of chronic immunosuppressive therapies.
Chimerism refers to a state wherein both the donor’s and the recipient’s hematopoietic stem cells (HSCs) coexist in the recipient’s bone marrow. It is also a potential study biomarker, predictive of the recipient tolerating the donated organ without the need for chronic immunosuppressants.
The next update on the FREEDOM-1 trial is expected in June.
FCR001 is being explored across multiple therapeutic applications.
— A phase II trial evaluating the potential of FCR001 to induce durable immune tolerance in patients who have previously received a kidney from a living donor (delayed tolerance), dubbed FREEDOM-2, is underway.
— Research is underway to test FCR001 in deceased donor kidney transplant.
— A phase II trial of FCR001 in scleroderma, its first severe autoimmune disease indication, dubbed FREEDOM-3, is ongoing.
Scleroderma is a rare, clinically heterogenous, progressive, multisystem, chronic autoimmune disorder that primarily affects the connective tissues.
Cash position:
The company’s cash on hand totaled $245 million as of December 31, 2021.
Talaris began trading on the Nasdaq Global Select Market, under the ticker symbol “TALS”, on May 7, 2021, pricing its shares at $17 each.
The stock has traded in a range of $6.17 to $19.82 in the last 1 year. TALS closed Friday’s trading at $7.10, down 1.66%.
Source: Read Full Article