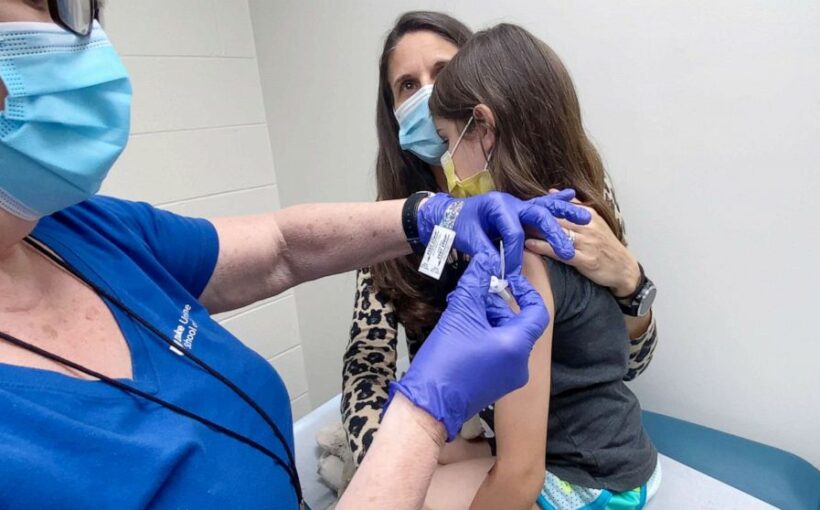
The Pfizer vaccine has been authorized for children ages 12 and up, widening the U.S. population that will be protected against the virus and bolstering chances for a safe return to full time school in the fall, the Food and Drug Administration announced Monday.
“Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations,” said acting FDA Commissioner Janet Woodcock.
Pfizer announced in late March that its clinical trials showed the vaccine was safe and 100% effective in children ages 12-15, similar to the 95% efficacy among adult clinical trial participants.
Pfizer’s vaccine was the first to show data in children as young as 12, a promising first step toward vaccinating younger Americans. The vaccine was the only option for those 16 and 17 and older in the U.S. with Moderna and Johnson & Johnson authorized for those 18 and older. Now, the FDA authorization means kids throughout middle and high schools will have the opportunity to be vaccinated before the fall, alleviating many of the hurdles schools face in stopping transmission.
Currently, about 115 million Americans are fully immunized — about 35% of the population.
After a trial with 2,260 children, Pfizer found no cases of infection among the children who had been given the vaccine and 18 cases of infection among the children who received a placebo. Real-world results often vary a bit from clinical trials.
According to Pfizer, the vaccine was also safe with adolescents experiencing a similar range of side effects as seen in older teens and young adults — generally seen as cold-like symptoms in the two to three days after the second dose.
In an interview with ABC News ahead of the authorization, Pfizer CEO Albert Bourla said getting children the vaccine would be “great news,” not only for the kids, “but also for the parent, grandfathers and therefore the American society in general.”
Bourla acknowledged that some parents might be nervous to vaccinate their young kids, but said Pfizer has been “extremely careful with children” and waited to begin studying the vaccine with anyone 15 or younger until hundreds of millions of doses were studied in adults.
“What we can promise to our parents is that we have done very thorough examination, more thorough than in any other vaccine, exactly because of the visibility that this vaccination is having,” Bourla told ABC News.
Moderna, the second mRNA vaccine approved for use in the U.S., is available for those 18 and up. The company said it is still studying the results among children ages 12 and up.
Dr. Ashish Jha, dean of the Brown University School of Public Health, said on ABC’s “Good Morning America” recently that getting vaccines to kids “will make an enormous difference for infection numbers in the community and schools for fall as well.” Currently, about 100 million Americans are fully immunized — about 30% of the population.
While children are not considered at high risk of severe illness from the coronavirus, they represent about a quarter of the U.S. population and vaccinating them is critical to stopping the spread of the virus.
Bourla also noted that kids can still spread the virus.
“When the kids are vulnerable, they are also contributing to the pool where the virus can replicate and, of course also, they are contributing to the chain of reinfections to other group ages,” he said.
“In addition to protecting the kids, as I said, the most important thing is that you can contribute to herd immunity in the country,” he added.
Experts predict the U.S. needs anywhere between 70% to 85% of the U.S. population to be vaccinated before it can reach herd immunity, when there is enough of a defense against the virus that it will no longer be able to spread. Specialists say the higher number of people with immunity the more difficult it is for the virus to spread, so every vaccinated person reduces the risk for those around them from getting COVID too.
Both Pfizer and Moderna are continuing to study the vaccines in trials of children ages 6 months to 11 years old. Bourla, the CEO of Pfizer, confirmed that the company is expecting to have data on vaccines for elementary school students by the end of the year.
ABC News’ Sony Salzman contributed to this report.
Source: Read Full Article