Shares of Fulcrum Therapeutics Inc. (FULC) have lost nearly 19% year-to-date and trade around $9.
Fulcrum Therapeutics is a clinical-stage biopharmaceutical company developing drugs for patients with genetically defined rare diseases in areas of high unmet medical need.
The company is all set to report data from its phase 1 trial in healthy adult volunteers with FTX-6058 for sickle cell disease in mid-2021.
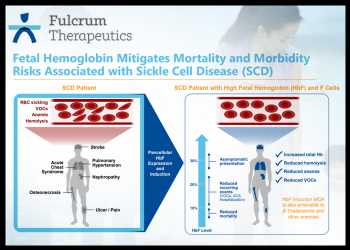
Sickle cell disease (SCD) is a genetic disorder of the red blood cells caused by a mutation in HBB gene, i.e., the gene that encodes a blood protein called beta globin. A key component of hemoglobin is beta globin. A mutation in the HBB gene disrupts the oxygen transport and results in the formation of red blood cells that have a sickle shape.
Some of the common symptoms of SCD are anemia, pain, infections, stroke, heart disease, pulmonary hypertension, kidney failure, liver disease and reduced life expectancy.
Novartis AG’s Adakveo, Global Blood Therapeutics’ Oxbryta, Emmaus Life Sciences’ Endari, and Hydroxyurea are some of the approved treatment options for SCD.
FTX-6058 as an oral therapeutic is designed to induce the expression of fetal hemoglobin (HbF) to compensate for the mutated adult hemoglobin in sickle cell disease, resulting in reduction or elimination of symptoms.
The phase I trial of FTX-6058, initiated last October, is comprised of four parts. Part A is a randomized, double-blind, placebo-controlled, single ascending dose (SAD) study in up to six cohorts. Part B is a randomized, double-blind, placebo-controlled, multiple ascending dose (MAD) study in up to four cohorts dosed once daily for 14 days. Part C is an open label pilot food effect study in subjects randomized to take FTX-6058 with and without a high-fat meal, and Part D, an open label study to evaluate the potential of FTX-6058 to induce CYP3A, an enzyme involved in the metabolism and clearance of drugs.
Last week, the company reported results from its phase 2b clinical trial of oral Losmapimod in 80 participants with facioscapulohumeral muscular dystrophy (FSHD), a disorder characterized by muscle weakness and wasting.
The phase IIb trial of Losmapimod, dubbed ReDUX4, failed to meet the primary endpoint, i.e., the change in DUX4-driven gene expression which was included as an experimental biomarker. However, Losmapimod showed statistically significant and clinically relevant benefit across multiple structural, functional and patient reported endpoints, say, decreased muscle fat infiltration, improved reachable workspace, and improved patient global impression of change.
The company plans to meet with health authorities, including the FDA, in the second half of 2021 to determine the regulatory path for Losmapimod in FSHD.
Cash Position:
As of March 31, 2021, the company’s cash, cash equivalents, and marketable securities totaled $143.9 million.
FULC has traded in a range of $7.01 to $21.44 in the last 1 year. The stock closed Tuesday’s trading at $9.51, up 5.55%.
Source: Read Full Article